We begin a three part series on the significance of quality in the manufacture and supply of herbal ingredients. Herein, our scientists unravel the intricacies of the quality chain, from raw material sourcing through finished ingredient evaluation. Sourcing and authentication of raw herbs is covered in the first part of this series. The role of quality control starts from the stage of identification of raw materials.
At our facilities, all herbal raw materials are procured from approved vendors after conducting vendor audit, to ensure the supply of consistent quality raw materials. We scrutinize the documentation of their quality system and storage facilities. In most cases a second supplier is also identified.
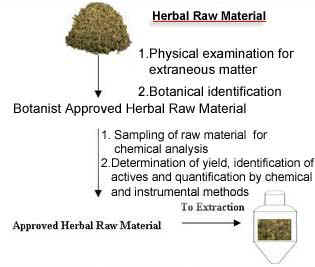
Firstly, botanicals are authenticated for genus and species, by a qualified botanist. An in-house herbarium facilitates the comparison of raw materials at any given time.
Once our botanist approves the herbal raw material, it is subjected to experimental extraction to test the yield, identity and quantity of actives, using sophisticated instrumentation (HPLC, GC, UV, IR). Raw materials will be used for commercial extraction only if they pass these tests, and conform to our specifications.
Product Ads
Look for Sabinsa product ads in the following publications this month HAPPI, Soap Perfumery & Cosmetics, Cosmetics & Toiletries, Natural Products Insider, Whole Foods Magazine, Nutrition Industry Executive, Functional Foods & Nutraceuticals, Nutraceuticals World and Nutracos.








